DfX in Action: Modular Strategies for Assembling, Servicing, and Optimizing Complex Optical Systems
Published
November 17, 2025
Written by Nathan Wallace
ABSTRACT
Design for Excellence (DFX) is a strategic approach that guides key decisions throughout the design process of complex optical systems. The “X” can represent various processes within manufacturing, assembly, cost, or performance. Explore how modular design techniques support assembly, serviceability, and system performance, offering real-world insights into practical implementation, design trade-offs, and system optimization. Applying DFX principles and modular techniques early in development can streamline processes, reduce costs, and elevate overall product quality. [1]
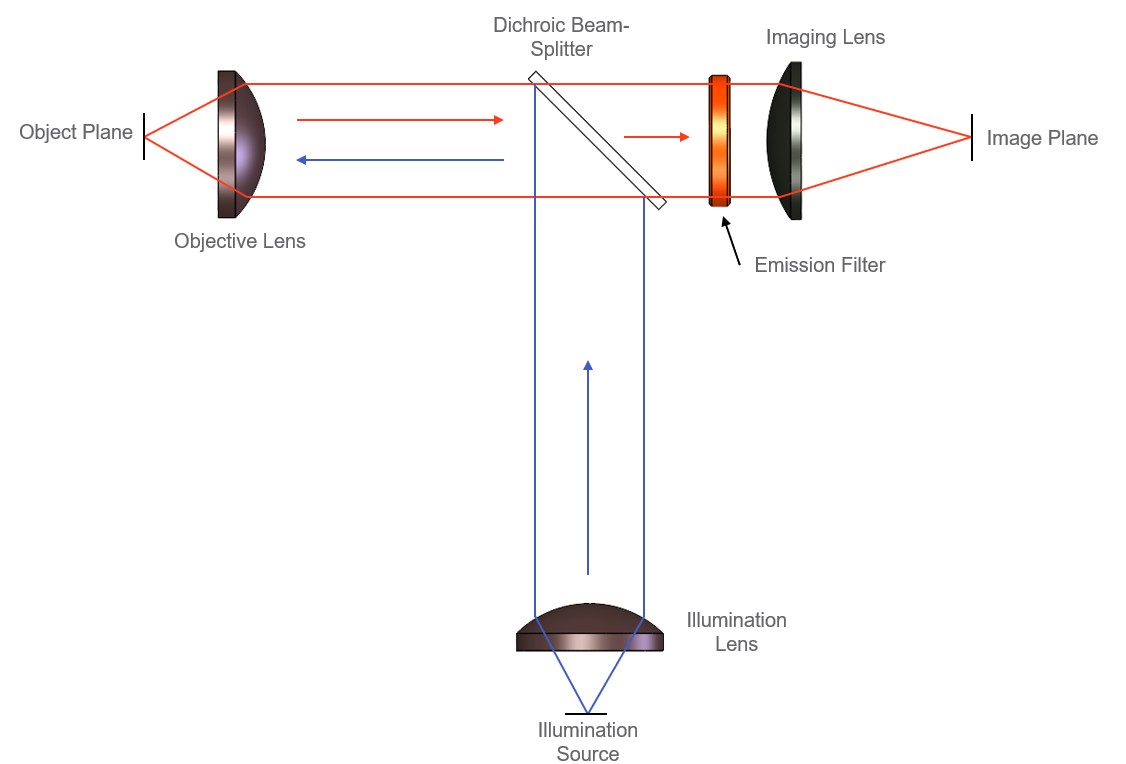
High-level optical architecture of a fluorescence imaging system. Excitation light (illumination source) is directed towards the dichroic beam-splitter, where it is delivered to the object plane through the objective lens. Emission light is collected by the objective lens, passes through the emission filter, and is imaged onto the image plane by the imaging lens.


About the Presenter:
Nathan Wallace leads the Engineering Services team at Optikos with a focus on opto-mechanical engineering. He contributed to numerous successful engineering projects across a wide field of applications, including aerial imaging lenses, microscopy systems, fluorescence imaging systems, and custom optical metrology instrumentation. Nathan received a Bachelor’s Degree in Mechanical Engineering from the University of Pittsburgh in 2006 and a Master’s Degree in Optical Sciences in 2021 from the University of Arizona.
View the Presentation Slide Deck and Technical Paper here:
References
[1] N. Wallace, “DfX in Action: Modular Strategies for Assembling, Servicing, and Optimizing,” in SPIE Optifab, Rochester, 2025.